ʻO ka ʻokoʻa koʻikoʻi ma waena o ka nitrate a me ka nitrite, ʻo ia ka nitrate i ʻekolu mau ʻāpana oxygen i hoʻopaʻa ʻia i kahi atom nitrogen a ʻo ka nitrite aia ʻelua mau mea oxygen i hoʻopaʻa ʻia i kahi atom nitrogen.
ʻO ka nitrate a me ka nitrite nā anion inorganic i loaʻa i nā ʻātoma nitrogen a me ka oxygen.Loaʻa i kēia mau anion ʻelua kahi hoʻoili uila -1.Loaʻa lākou ma ke ʻano he anion o nā pūhui paʻakai.Aia kekahi mau ʻokoʻa ma waena o ka nitrate a me ka nitrite;e kūkākūkā mākou i kēlā mau ʻokoʻa ma kēia ʻatikala.
He aha ka Nitrate?
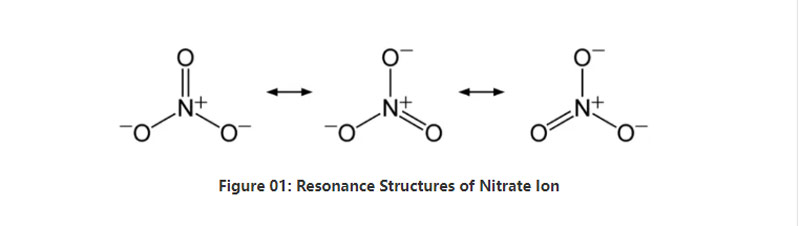
ʻO ka Nitrate he anion inorganic nona ke ʻano kemika NO3–.He anion polyatomic nona nā ʻātoma 4;hoʻokahi ʻātoma nitrogen a ʻekolu mau ʻātoma oxygen.Loaʻa i ka anion -1 ka uku holoʻokoʻa.ʻO 62 g/mol ka nui mola o kēia anion.Eia kekahi, loaʻa kēia anion mai kona ʻakika conjugate;nitric acid a i ʻole HNO3.ʻO ia hoʻi, ʻo ka nitrate ke kumu conjugate o ka waikawa nitric.
ʻO ka pōkole, loaʻa i ka ion nitrate hoʻokahi ʻātoma nitrogen i waenakonu e hoʻopaʻa ʻia me ʻekolu mau ʻātoma oxygen ma o ka hoʻopaʻa ʻana kemika covalent.I ka noʻonoʻo ʻana i ke ʻano kemika o kēia anion, loaʻa iā ia ʻekolu mau paʻa NO like (e like me nā ʻano resonance o ka anion).No laila, ʻo ka geometry o ka mole he planar trigonal.Lawe ʻia kēlā me kēia ʻātoma oxygen i kahi − 2⁄3 hoʻopiʻi, e hāʻawi ana i ka uku holoʻokoʻa o ka anion e like me -1.
Ma ke kaomi maʻamau a me ka mahana, aneane pau nā pūhui paʻakai i loaʻa kēia anion i ka wai.Hiki iā mākou ke ʻike i nā paʻakai nitrate kūlohelohe ma ka honua ma ke ʻano he waihona;nā waihona nitratine.Loaʻa ka nui o ka sodium nitrate.Eia kekahi, hiki i ka bacteria nitrifying ke hana i ka ion nitrate.ʻO kekahi o ka hoʻohana nui ʻana o nā paʻakai nitrate i ka hana ʻana i nā mea kanu.Eia kekahi, pono ia ma ke ʻano he oxidizing agent i nā mea pahū.
He aha ka Nitrite?
ʻO ka Nitrite he paʻakai ʻanoʻano nona ke ʻano kemika NO2–.ʻO kēia anion he anion hoʻohālikelike, a he hoʻokahi ʻātoma nitrogen i hoʻopaʻa ʻia i ʻelua mau ʻātoma oxygen me nā mea paʻa kemika NO covalent ʻelua.No laila, aia ka atom nitrogen i waenakonu o ka mole.Loaʻa i ka anion -1 ka uku holoʻokoʻa.
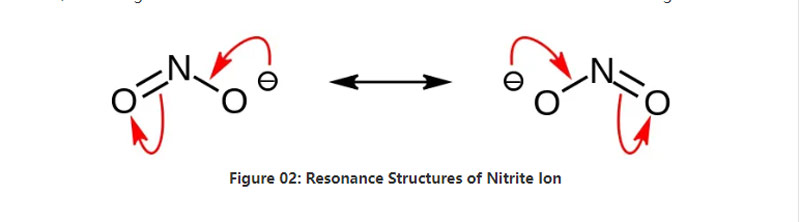
He 46.01 g/mol ka nui mola o ka anion.Eia kekahi, loaʻa kēia anion mai ka waikawa nitrous a i ʻole HNO2.No laila, ʻo ia ke kumu conjugate o ka waikawa nitrous.No laila, hiki iā mākou ke hana i nā paʻakai nitrite ma ka ʻoihana ma o ka hoʻolilo ʻana i ka uahi nitrous i loko o ka solution sodium hydroxide wai.Eia kekahi, hana kēia i ka sodium nitrite hiki iā mākou ke hoʻomaʻemaʻe ma o ka recrystallization.Eia kekahi, pono nā paʻakai nitrite e like me ka sodium nitrite i ka mālama ʻana i ka meaʻai no ka mea hiki ke pale i ka meaʻai mai ka ulu ʻana o ka microbial.
He aha ka ʻokoʻa ma waena o ka Nitrate a me ka Nitrite?
ʻO ka Nitrate he anion inorganic i loaʻa ke ʻano kemika NO3– akā ʻo ka Nitrite he paʻakai inorganic nona ka formula kemika NO2–.No laila, aia ka ʻokoʻa nui ma waena o ka nitrate a me ka nitrite ma luna o ka hoʻohui kemika o nā anion ʻelua.ʻo ia;ʻO ka ʻokoʻa koʻikoʻi ma waena o ka nitrate a me ka nitrite ʻo ia ka nitrate i ʻekolu mau ʻātoma oxygen i hoʻopaʻa ʻia i kahi ʻātoma nitrogen a ʻo ka nitrite i loko o ʻelua mau mea oxygen i hoʻopaʻa ʻia i kahi ʻātoma nitrogen.Eia kekahi, loaʻa ka ion nitrate mai kona ʻakika conjugate;ka nitric acid, aʻo ka nitrite ion i loaʻa mai ka waikawa nitrous.E like me kekahi ʻokoʻa koʻikoʻi ma waena o nā ion nitrate a me nā nitrite, hiki iā mākou ke ʻōlelo ʻo ka nitrate he mea hoʻoheheʻe ʻia no ka mea hiki ke hoʻemi wale ʻia ʻoiai hiki ke hana ʻo nitrite ma ke ʻano he mea hoʻokahe a hoʻemi.
Ka manawa hoʻouna: Mei-16-2022





